Abstract
We demonstrate magnesium zinc oxide nanostructure (MZOnano) modified multifunctional devices for the full-scale dynamic monitoring of Pseudomonas aeruginosa (P. aeruginosa) biofilm formation: the dual-gate thin film transistor (DGTFT) as an electrical sensor for early stage detection and the quartz crystal microbalance (QCM) as an acoustic sensor for long-term monitoring. The sensing surfaces of both devices were modified with MZOnano to enhance their sensitivity and biocompatibility. P. aeruginosa bacteria were cultured in vitro on both sensing surfaces. The early stage detection is realized by sensing the charge transfer from cell membrane to the MZOnano during bacterial adhesion using the DGTFT biosensor while the monitoring of the long-term evolution is achieved through the sensing of mass loading and viscoelastic transition during biofilm development using the MZOnano QCM. The drain current of DGTFT starts to change at the beginning of the test and levels off after ∼6.5 h of bacterial inoculation, whereas the signals of MZOnano QCM become detectable after ∼5 h and then lasts for 24 h. The full-scale process of biofilm development covering from bacterial adhesion to maturation is thus dynamically monitored using this MZOnano modified multifunctional sensing technology.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Pseudomonas aeruginosa (P. aeruginosa) is a Gram-negative opportunistic pathogen that causes severe chronic nosocomial infections, particularly in immunocompromised patients. P. aeruginosa biofilms form readily on the surface of implants and indwelling devices, such as urinary catheters and ventilator tubes, resulting in serious risks to patients. 1 It is even worse that such biofilm-associated infections are often difficult to treat and thus frequently life-threatening.
The case fatality rate for patients infected with P. aeruginosa is close to 50%, 1 mainly due to two reasons: (i) the onset of its biofilm formation is difficult to detect with routine clinical methodologies, and (ii) once the biofilms are established, the strong effect of antimicrobial tolerance by biofilms often leads to long-term high dose antibiotic treatments or even repeated surgical procedures to eradicate the biofilms.
The formation of biofilms generally takes place in three phases. It begins with the attachment of planktonic bacterial cells at the sites of infection. Then the bacterial microcolonies expand through cell division and extracellular polymeric substance (EPS) production. Finally, it forms matured biofilms. Knowledge of the dynamic behavior and characteristics of the biofilms of a particular bacterial strain in all three phases is highly desirable for studying and the treatments of biofilm infections, as well as for developing the coating materials that prevent biofilm formation. To better understand the entire process of P. aeruginosa biofilm formation, a full-scale dynamic biosensing technology is desirable. First, the early stage (i.e. bacterial adhesion stage) detection of biofilm formation is critical, because the sooner the onset of biofilm formation is detected, the more effective the prevention of infection will be. Once passed the early stage, its growth kinetic profile should also be monitored during the long-term evolution of biofilm formation because such information can serve as a feedback signal.
Various approaches have been used to detect P. aeruginosa bacterial adhesion and its biofilm formation process. S. Kim et al. demonstrated the detection of bacterial adhesion using electrochemical impedance spectroscopy 2 in which the cell adhesion was monitored in the first hour of bacterial inoculation, using percentage change of electrical double layer capacitance as the output signal. Although this work suggested the possibility of using their method for detecting the initial P. aeruginosa biofilm formation, no experimental demonstration was performed. Moreover, the technology was not used for monitoring biofilm development in its subsequent stages. A surface acoustic wave (SAW)-based sensor was used to monitor the long-term evolution of P. aeruginosa biofilm for 24 h to evaluate the efficacy of biofilm treatment. 3 However, the mass-based SAW sensors are mainly used for the long-term monitoring of biofilm formation, not for the early stage detection, because the tiny mass loading in the early stage is below the detection limit of this technology. Some effort has also been made to realize both functions in a single device. For example, cyclic voltammetry was used to differentiate bacterial attachment and biofilm formation through their different signal responses. 4 In the case of bacterial attachment, cell attachment to the electrodes results in an increase of current in the cyclic voltammograms due to bacterial metabolism activities, whereas in the case of biofilm formation, a decrease of current occurs due to the reduction of electrode surface area. However, the measurements of bacterial adhesion and biofilm formation were separately conducted by controlling the nutrient supply, thereby not representing a true dynamic and real-time monitoring of the full-scale development of biofilm formation. For clinical applications, a full-scale continuous monitoring technique is needed to guide the treatment of biofilm infections promptly and effectively.
ZnO and its ternary alloy compound magnesium zinc oxide (MgxZn1−xO, i.e. MZO) are wide energy bandgap semiconductor materials that are used as the active channel layers for thin film transistors (TFTs). However, pure ZnO suffers from thermal instability and negative bias stress instability due to the existence of intrinsically formed defects, including oxygen vacancies. 5,6 Such instabilities can severely impact the electrical performance of the device. A small amount of Mg can help suppress the oxygen vacancies benefit from stronger Mg–O bonding, and therefore MZO is used as an optimized channel material over ZnO for TFTs. MZO TFTs have been employed in a wide range of applications, such as photodetectors for UV lights, 7 converters/inverters for building-integrated photovoltaics (BIPV), 8,9 and frequency modulators for SAW devices. 10
ZnO- and MZO-based nanostructures (ZnOnano and MZOnano) are known to be functional and biocompatible nanostructures for biosensing applications. ZnOnano can be grown with different surface morphologies on various substrates, including Si, glass, and metal. 11–13 ZnOnano possesses a large effective surface, leading to high sensitivity for sensing. Through the proper UV light illumination, the ZnOnano surface exhibits superhydrophilic property, which enhances the surface immobilization and reduces the consumption of liquid samples. 14 By doping a small amount of Mg into the ZnOnano, the resultant MZOnano can work in a wide pH range of biochemical environment during bio-measurement. 15 The strong Mg–O bonding also minimizes the release of Zn2+ ions from MZOnano to the culture medium, thereby reducing Zn2+ ion toxicity to bacterial cells. 16,17
In this work, we report the dynamic monitoring of the full-scale process of P. aeruginosa biofilm formation using multifunctional sensing technology. The technology consists of an MZO DGTFT electrical sensor and a QCM acoustic sensor. Both devices use the same MZOnano as the sensing layer to enhance their sensitivity. The MZO DGTFT with an extended MZOnano modified gate detects the onset of biofilm development utilizing the electrical charge transfer mechanism, whereas the MZOnano modified QCM is a bulk acoustic wave device that monitors the kinetic growth profile of the subsequent developing stages through mass accumulation and viscosity transition. We have previously demonstrated that the DGTFT biosensor enables to detect the early stage formation of Staphylococcus epidermidis biofilms. 18 During the initial bacterial adhesion stage, the charge transfer phenomenon between bacterial cells and the MZOnano coated gate electrode signals the onset of biofilm formation. However, DGTFT is not capable of detecting the subsequent changes of biofilm development beyond the early stage because the electrical charge transfer is just an interfacial phenomenon. To remedy this shortcoming, the MZOnano modified QCM biosensor is used in conjunction with the DGTFT biosensor. QCM is a bulk acoustic wave (BAW) device with high sensitivity to mass loading and viscosity change. In 2010, Chen et al. monitored the long-term behaviors of the Pseudomonas fluorescens biofilm development using QCM. 19 However, during the early stage of biofilm formation, the mass loading is too small to generate detectable output signal response. In contrast, the electrical charge transfer during the early stage of biofilm development (i.e. bacteria adhesion) is much more significant than that of the mass loading. The unique features of DGTFT and QCM complement each other. Dynamic monitoring of the full-scale development of biofilm formation can thus be realized by combining these two technologies: DGTFT for the early stage charge detection while QCM for the mass loading and viscosity change for long-term monitoring. Furthermore, both devices use the same critical MZOnano. The MZOnano was grown on the sensing area of both DGTFT and QCM, serving as the biomolecule interface via surface modification to enhance sensitivity. Biofilms are incubated simultaneously on the MZOnano of both sensing devices with the same culture conditions. Through signal acquisition and processing, the measurement results are reported continuously as the function of time.
This multifunctional biosensing technology is capable to dynamically detect the details of the biofilm formation process, ranging from bacterial adhesion at the early stage to biofilm maturation at the final stage. In clinical applications, such hybrid technology should benefit the in vitro study of biofilm formation on implantable devices and help medical professions promptly treat patients with effective therapies.
Experimental
Design of the hybrid sensing technology: MZO DGTFT + MZOnano QCM
A schematic description of this hybrid biosensing system is presented in Fig. 1. Figure 1a depicts the process of biofilm development. The formation of biofilms has three stages: bacterial adhesion (early stage), bacterial expansion (growth stage), and biofilm maturation (final stage). Figure 1b shows the schematic diagram of the sensing system. It consists of two biosensors: (i) DGTFT for the early stage detection of biofilm formation, and (ii) QCM for the long-term dynamic monitoring for the subsequent biofilm development. The sensing surfaces of both devices were modified with the same MZOnano layer. Then they were immersed in the bacterial incubator. The full-scale process of P. aeruginosa biofilm development was monitored continuously using this complementary biosensing technology.
Figure 1. (a) Outline of the biofilm development process: bacterial adhesion, bacterial expansion, and biofilm maturation. (b) A schematic of the hybrid and multifunctional biosensing system consisting of the MZO DGTFT with an extended MZOnano gate for detecting bacterial adhesion (early stage) and the MZOnano QCM for monitoring the subsequent stages.
Download figure:
Standard image High-resolution imageFabrication of MZO DGTFT biosensor
The MZO DGTFT biosensor has an MZO DGTFT as the signal transducer and an MZOnano modified sensing pad as the biological receptor. The detailed fabrication process of the DGTFT biosensor was described elsewhere. 18 Here, we briefly summarize it as follows: first, a 50 nm Cr bottom gate layer was deposited and patterned on a 0.4 mm thick commercial glass substrate (PG&O), after which a 100 nm thick SiO2 bottom gate dielectric was formed by plasma enhanced chemical vapor deposition (PECVD) at 250 °C. Next, a 5 nm ultra-thin MgO diffusion barrier 8,10 followed by a 40 nm Mg0.03Zn0.97O active channel layer were deposited sequentially by metal oxide chemical vapor deposition (MOCVD) at ∼400 °C and fixed at width/length (W/L) = 160 μm/15 μm by wet etching. DeZn (Diethylzinc), MCp2Mg (Bis-(methylcyclopentadienyl)magnesium), and ultra-high purity (99.999%) oxygen gas were used as the Zn metalorganic source, Mg metalorganic source, and oxidizer, respectively. Then, the source/drain electrode (85 nm Ti/35 nm Au/5 nm Ti) was formed using the lift-off process. The SiO2 top dielectric was subsequently deposited using PECVD with a thickness of 70 nm at the same temperature. A 150 nm thick Al top gate electrode was then formed by the electron beam evaporator and wet etching. VIA openings were finally made using diluted buffered oxide etch solution.
The fabrication process of the extended sensing pad started with the deposition of a 5 nm Cr/ 50 nm Au metal layer as the conducting electrode on the same kind of glass substrate. Then, the metal layer was etched to form squares of 120 mm2 and followed by the growth of MZOnano using MOCVD. Finally, the nanostructured layer was patterned and wet etched, while leaving a small area of the metal layer exposed for electrical contact.
The left side of Fig. 1b shows the cross-sectional schematic drawing of the MZO DGTFT and its testing configurations. During testing, the top gate of DGTFT was electrically connected to the exposed electrode of the extended sensing pad. The sensing pad was immersed in the bacterial incubator.
Fabrication of MZOnano QCM
The MZOnano QCM device consists of an MZOnano modification layer that is integrated with a commercial AT-cut QCM (International Crystal Manufacturing, Inc.) by growing them directly on the surface of the sensing electrode of the QCM using MOCVD. The growth conditions of the MZOnano are described below. A schematic drawing of the top and cross-sectional multilayer view of the MZOnano QCM is shown on the right side of Fig. 1b. The standard QCM's quartz layer is sandwiched by two 100 nm gold electrodes. The sensing area of the QCM is 20.47 mm2. The operation frequency of the standard QCM is 10 MHz, while the MZOnano QCM has an operating frequency of 9.912 MHz.
Growth of the MZOnano modification layer on sensing surfaces
The MZOnano layers were deposited on the top electrodes of QCM and the extended pad of DGTFT to serve as the sensing surfaces to enhance their sensitivity. The 400 nm thick Mg0.04Zn0.96O nanostructured films were grown using MOCVD at ∼500 ℃ and the chamber pressure was maintained at ∼60 Torr. 20 DeZn, MCp2Mg, and high purity oxygen gas were used as the precursors and oxidizer, which are the same as the ones used for the DGTFT's MZO channel layer growth. Then the MZOnano surfaces underwent UV illumination to achieve superhydrophilic characteristics to enhance the sensitivity and reduce the consumption of liquid biological samples. 21 The nanostructured surface provides a giant effective sensing area for bacteria to attach. In addition, as bacteria prefer to start adhesion at somewhere sheltered from shear forces, 22 the proper surface roughness also provides such optimum condition.
Biological samples and protocols
Bacterial biofilm incubation
P. aeruginosa PAO1 was inoculated into Mueller Hinton Broth (MHB; Fisher Scientific) and grown at 37 °C for 16−18 h in test tubes placed in a shaking incubator operating at 200 rpm. The stationary phase cultures were diluted 100-fold into the fresh MHB medium and then pre-loaded in the Teflon cell culture well. The extended MZOnano sensing pads of DGTFT and the MZOnano QCMs were sterilized and placed into the wells. The biofilms were grown in the wells and the growing process was monitored by the DGTFT and the MZOnano QCM.
Crystal violet staining
We used the crystal violet staining to verify biofilm formation on the MZOnano surfaces. MZOnano was deposited on glass substrates and then divided into two sets: one for control and one for testing. Both sets were prepared through the same procedure as outlined above, however, for the control set the MZOnano glass substrates were incubated with only MHB growth medium lacking bacteria. The separate Petri dishes containing the control and testing sets each containing multiple samples were placed in a static incubator at 37 °C to induce biofilm formation. At various time points during incubation, one sample each from the control and testing sets was retrieved for crystal violet staining. The time points of retrieval were 0, 1.7 h (100 min), 3.3 h (200 min), 5 h (300 min), 8 h, 15 h, and 24 h, which covers the biofilm development at different times. After removal from the culture, the samples were washed three times with 5 ml of 0.9% NaCl solution to remove planktonic cells to assure that only the biofilms were attached to the sample surface. Biofilms attached on the sensing surface were then stained with 0.2% crystal violet for 10 min, followed by washing three times with 0.9% NaCl. The microscopic images were then taken for each time point.
Measurement and signal processing
Measurement and parameters extraction for MZO DGTFT biosensor
In the first step of measurement, the basic electrical characteristics of the MZO DGTFT were examined. The transistor's transconductance plots (i.e. drain current IDS vs bottom gate biasing voltage VBG) were recorded under various top gate biasing voltages VTG. Then, the control experiment with MHB medium only was tested three times for 660 min. The results showed an average drain current variation of 1.6% with an average standard deviation of 2.9%. Comparing with the actual biosensing results which will be discussed later, such background variations are negligible. Next, the transfer characteristic variations, as a result of the early stage detection of P. aeruginosa biofilm formation, were obtained. As the electrons from bacterial membrane transfer to the MZOnano, an equivalent micro biasing voltage is applied to the top gate of the DGTFT. Such top gate bias changes the conducting current IDS flowing through the channel layer through the electric field effect. By extracting the current variations under proper bottom gate biasing VBG, the optimized signal output can be realized with the best combination of high sensitivity and stability. At last, the time-dependent signal response with standard deviation error bars is presented in combination with the QCM's results to form the full-scale monitoring results.
Throughout the experiments, the MZO DGTFT was placed inside a light-tight measurement station. Its top gate was electrically connected to the extended MZOnano sensing pad where the bacterial incubation took place. The electrical measurements were carried out using the HP-4156C semiconductor parameter analyzer. The VBG was swept between −5 V to 15 V to find the optimum operation condition. The voltage across source and drain VDS acts as an electron pump to drive current IDS across the channel and was maintained at 0.1 V to minimize power consumption. The device threshold voltage VTH was extracted using the linear fitting method for 10%−90% of the maximum drain current. The subthreshold slope S.S value was extracted from a 3-decade range in the sub-threshold region (IDS = 10−12 − 10−9 A) of the transconductance curve in logarithm scale.
MZOnano QCM measurement and data analysis
The MZOnano QCM was used to monitor the long-term (24 h) development process of P. aeruginosa biofilms. The device was placed in a standard bacterial incubator with a controlled ambient environment. The characterization of MZOnano QCM was conducted using an HP-8573D network analyzer, which was connected via the IEEE-488 general purpose interface bus (GPIB) to the universal serial bus (USB) of a microprocessor running of a LabView data acquisition program. The impedance transmission spectrum Z21 (ω) of the device was automatically measured at fixed time intervals and digitally stored. The MZOnano QCM was sterilized and deployed inside a Teflon cell-growth well seeded with growth media. Both frequency shift and motional resistance keep slightly increasing until equilibrium at 40 min. Then, P. aeruginosa cells were added to the device. Background signal variations were excluded during the biosensing process.
The impedance spectrum of the MZOnano QCM can provide the biophysical properties of the bacterial culture as a function of time. The spectrum features two important parameters, namely the peak frequency shift (Δf) and the motional resistance (Rload). The frequency shift parameter Δf of the MZOnano QCM is determined by measuring the absolute value of the difference in resonant frequency between the case without and with mass loading. 23
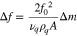
where for Eq. 1, Δf = f0 − f(t) is the frequency shift and f0 is the intrinsic operating frequency of the device, υq and ρq are respectively the acoustic velocity and mass density of the AT-cut quartz layer, A is the sensing area of the top electrode, and Δm is the mass change, i.e. the accumulated mass on the sensing electrode.
The motional resistance Rload is obtained from the real part of the complex amplitude in the impedance transmission spectrum, i.e. Rload = Re{Z21(ω)}. With the development of biofilm on the MZOnano QCM sensing surface, the biofilm undergoes viscoelastic transitions due to the attachment and metabolism activities of bacterial cells and the formation of EPS. These viscoelastic transitions manifest themselves as modulations in the amplitude of the impedance transmission spectrum, specifically on Rload. These two parameters can be represented by the equations below, respectively. 13,24
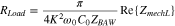
where K2 is the coupling coefficient of the piezoelectric layer, ω0 is the resonant angular frequency of the QCM with no mass loading, C0 is the capacitance of the device, ZBAW is the impedance of the device without the mass accumulation, and ZmechL is the mechanical load impedance due to the biofilm.
Results and Discussion
We started with the characterizations of the electrical performance of DGTFT, followed by the extraction of signals that represent the status of early stage biofilm formation. Next, the full-scale dynamic signal response resulted from P. aeruginosa biofilm formation is demonstrated, where the early stage signal was detected by DGTFT biosensor and the long-term follow up process was monitored by MZOnano QCM. For comparison, crystal violet staining was used to show the microscopic images of biofilm development at different incubation times.
Biosensor operation
Electrical characteristics of MZO DGTFT biosensor
The transfer characteristics of the MZO DGTFT were firstly tested with its top gate electrode electrically connected to a DC power supply. We chose VTG from 0 to −1 V with a step of −0.2 V as the setting to demonstrate the highly sensitive signal of the device in response to the voltage alternation on its top gate electrode. Such VTG was chosen because the bacteria tend to donate only a small fraction of their membrane electrons to the supporting substratum, 25,26 and thus the equivalent top gate bias induced by bacterial adhesion should be negative and small.
The measured results are shown in Fig. 2a. The curve at VTG = 0 V shows the threshold voltage of 7.47 V, and subthreshold slope of 637 mV dec−1. The low threshold voltage ensures the device with low power consumption. The steep subthreshold slope enables high sensitivity owing to the high electrical signal gain of the device. To have a closer look at the variations of the I-V curves due to different top gate biases, the inset figure in Fig. 2a shows the detailed characteristics in the range of -3 V < VBG < 2 V where the device is just about to be turned on. It could be clearly seen that these transfer curves exhibited parallel right shifts with respect to the increasing values of VTG. The threshold voltage VTH positively shifts ∼10% from 7.47 V at VTG = 0 V to 8.25 V at VTG = -1 V. Such right shifting can be explained as follows:
MZO is a kind of n-type semiconductor material. The bottom gate bias introduces a vertical electrical field that accumulates electrons at the bottom channel/dielectric interface. However, the negative top gate voltage partially depletes the accumulation channel. To compensate the depletion and turn on the device, the threshold voltage must be adjusted by an equivalent positive shift as shown in Fig 2.
Figure 2. (a) The electrical transfer characteristics of MZO DGTFT were tested with the top gate electrode connected to various top gate biases. The inset shows the detailed characteristics with VBG ranging from −3 V to 2 V. The I-V curves keep right shifting with the increasing values of negative top gate bias owing to the electrostatic field-effect. (b) The electrical signal response of the MZO DGTFT biosensor (2.5 V < VBG < 7.5 V) with its top gate electrically connected to the sensing pad where bacterial growth occurred. Drain current keeps decreasing as the incubation time increasing until t = 390 min.
Download figure:
Standard image High-resolution imageAn equation (Eq. 3) describing the relationship between VTH and VTG was proposed by G. Baek et al. 27
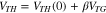
where VTH(0) denotes the threshold voltage when VTG = 0, and β is the coefficient related to the capacitance of dielectric layers and the channel layer. Based on the measurement result, the β value is calculated to be 0.78.
The right shifting of threshold voltage also lowers the drain current under a certain bottom gate biasing condition, especially in the triode region of the transfer characteristics. For example, the drain current at VBG = 0 V decreases nearly one decade from 1.37 × 10−9 A at VTG = 0 V to 1.41 × 10−10 A at VTG = −1 V (shown in the inset of Fig. 2a). The steep slope in the triode region of the transfer characteristics results in large current variations within a small range of bottom gate biases, and hence provides high current amplification when the top gate bias varies. Comparing to the threshold voltage change, the drain current change of DGTFT is particularly favorable for using as the signal to represent the early stage development of biofilm formation and is used to define the sensitivity of the biosensor.
The high electrical sensitivity of the device enables the DGTFT to detect the early stage of biofilm formation. Next, the MZO DGTFT biosensor was used to detect the onset of P. aeruginosa biofilm formation with its top gate electrode electrically connected to the sensing pad where bacterial growth occurs. Three MZOnano modified sensing pads were prepared. The detection of P. aeruginosa biofilms was performed on each of these pads using the same DGTFT device under the same microbial culture and measurement conditions. The sensing pad was immersed in the MHB medium and was allowed its baseline signal to stabilize before the P. aeruginosa culture solution was introduced at time t = 0. The measurements of transfer characteristics were made sequentially for a total of 900 min of incubation time.
In obtaining the signal that represents the status of early stage biofilm development, we've described how to balance between sensitivity and stable operations when choosing the best point of operation. 18 Using a similar method, VBG = 5 V is determined as the optimum operation point in this study. For better visualization, part of the transfer characteristics (2.5 V < VBG < 7.5 V) of a single set of measurements are shown in Fig. 2b. As can be clearly seen from the figure, the drain current keeps dropping until t = 390 min where it exhibits no significant variation comparing with the one at t = 900 min DGTFT is a highly sensitive electrical device. Drain current drops as a result of the negative bias applied on its top gate electrode. In the biosensing experiment, the current change is attributed to the electron charge transfer resulted from the bacterial adhesion to the MZOnano surface during the early stage development of the biofilm formation. 25,26
The testing results show no significant signal variation after t = 390 min, indicating limited long-term monitoring capability of the device. Thus, the biofilm development process beyond the early stage is essentially difficult to detect using this MZO DGTFT biosensor. This prompted us to employ the MZOnano QCM for monitoring the long-term biological evolutions of biofilm formation to complement MZO DGTFT for monitoring the later stages of biofilm formation.
Full-scale dynamic monitoring of biofilm development and formation
In this section, we present the full-scale dynamic monitoring results of the biofilm development and formation. The early stage detection capability of the MZO DGTFT biosensor is discussed above. Owing to the high electrical signal gain provided by the active device, its drain current variation as a result of bacterial charge transfer is utilized as the output signal to realize the early stage detection of biofilm formation. However, our results show that the signal saturates after a certain period. On the other hand, MZOnano QCM is capable of precisely measuring mass accumulation and viscoelastic transition on its surface, and thus provides high sensitivity of monitoring the subsequent stages of biofilm evolution.
To obtain the full-scale dynamic profile of biofilm evolution, both MZO DGTFT and MZOnano QCM biosensors were used to monitor the progress of biofilm development under the same microbial conditions.
The time-evolving signal response of the MZO DGTFT and the MZOnano QCM are plotted in Fig. 3. Percentage change of drain current is used as the signal of the DGTFT biosensor to represent the early stage development of biofilms. As mentioned above, VBG = 5 V is determined as the optimum operation point. No lag phase is exhibited by the bacterial culture, which can be attributed to having the bacterial culture introduced already in a metabolically active state and does not require time to enter cell division. 28 The percentage change of drain current keeps increasing as incubation time goes from t = 0, after which the currents level off at about t = 6.5 h (390 min), which signifies the detection point for the onset of biofilm formation. Although the biofilms were still growing and far from maturation at t = 6.5 h, as will be shown in the microscopic images, the charge transfer effect between the bacterial cell surface and the substratum surface was being gradually prohibited and diminished. The charge transfer only takes place at the interface between bacterial cells and the substrate because the biofilms of P. aeruginosa are poorly conductive. 29,30 After t = 6.5 h, the slope of the current change is approaching zero and the signal eventually becomes independent of time. The signal of DGTFT here demonstrates a maximum current change of 57% with an average standard deviation of 5.1%. Such early stage detection could alert medical professionals to treat the biofilms in a timely manner.
Figure 3. The signal variations during the full-scale development of P. aeruginosa biofilm formation, represented by the percentage change of drain current of DGTFT, motional resistance and frequency shift of MZOnano QCM.
Download figure:
Standard image High-resolution imageThe MZOnano QCM's resonance frequency shift Δf and the motional resistance Rload are also plotted in Fig. 3 with the same time axis as the DGTFT's results. The average standard deviations of Δf and Rload are 2.47 KHz and 11.68 Ω, respectively (they are too small to be shown in the figure scale). As shown in the figure, the QCM barely detects the early stage, especially before t = 5 h. The small mass and viscoelastic transition the sensor can detect limit its sensitivity for the early stage biofilm monitoring. The MZO DGTFT biosensor, however, already exhibits a 52% drain current reduction at t = 5 h when VBG = 5 V. Large increments in the QCM signal values start to be observed after t = 5 h. The Rload plot shows a rapid growth phase from t = 5 h to 15 h, but the signal stops increasing and saturates at about 15 h with a maximum change of ΔRload = 560 Ω. The motional resistance saturation indicates that the viscosity of the biofilms reaches its peak value although the biofilms are still increasing in mass as shown in the frequency shift data. The frequency shift Δf exhibits a steady increase all the way towards to the end of the experiment (t = 24 h) and reaches 160 KHz, indicating that the biofilms are still evolving and keep gaining weight during the course of development.
The MZO DGTFT biosensor shows a significant signal variation (52%) in the first 5-hour of biofilm development, but the signal diminishes after a certain period. The MZOnano QCM has difficulties in detecting the initial formation of biofilms but shows the ability to monitor the long-term later process of biofilm development, which is represented by two important characteristics of MZOnano QCM: frequency shift and motional resistance. The change of both parameters demonstrates the development of biofilms, whereas the saturation of motional resistance corresponds to the final biofilm maturation stage as will be shown below.
Microscopy characterization of crystal violet stained biofilms
Crystal violet staining assay was used to verify the formation of P. aeruginosa biofilms and traced the process at different times. The MZOnano modified glass substrates were used as the supporting surfaces for biofilm incubation. The same growth conditions were applied as used for biosensing. The optical microscopic images of the biofilm formation process on the MZOnano coated glass were taken at times of t = 0, 1.7 h (100 min), 3.3 h (200 min), 5 h (300 min), 8 h, 15 h, and 24 h as shown in Fig. 4.
Figure 4. Optical microscopic images of crystal violet stained P. aeruginosa biofilm formation on MZOnano modified glass substrate at different times.
Download figure:
Standard image High-resolution imageFrom the microscopic images in Fig. 4, we can hardly distinguish if there is bacterial adhesion on the sensing pad when t = 1.7 h. However, the result of the MZO DGTFT biosensor does show the obvious current reduction of 21% at t = 1.7 h, indicating bacterial adhesion. Afterward, it can be clearly seen that more bacterial microcolonies were formed at the sites of adhesion from t = 3.3 to 5 h and the biofilms began to take shape at t = 8 h. However, t = 8 h is the time point where the DGTFT already exhibits a signal saturation value of 57%. With the increase of incubation time, the microcolonies shown at t = 8 h kept developing and showed darker color when time reached t = 15 h. During this time period, the DGTFT biosensor does not show signal variation whereas the MZOnano QCM does show obvious changes in both the frequency shift and motional resistance. At t = 15 h, a set of size and shape of biofilms can be seen now, and biofilms at this stage are referred to as being "mature". 31 This is also the point where the motional resistance signal of MZOnano QCM reaches plateau. Finally, the biofilms covered the majority of the surface (∼64%) when t = 24 h.
Conclusions
We have demonstrated a multifunctional sensor technology for the full-scale detection of P. aeruginosa biofilm formation, ranging from bacterial adhesion to biofilm maturation. The detection system consists of an MZO DGTFT electrical sensor and an MZOnano QCM acoustic wave sensor. Both devices use the same MZO nanostructures as the sensing surface to achieve high sensitivity and biocompatibility. The two devices function in the complementary mode. The MZO DGTFT with the extended MZOnano gate pad detects the early phase biofilm formation through the charge transfer mechanism. Drain current starts varying at the beginning of the test and its percentage change reaches 52% at t = 5 h. On the other hand, the MZOnano QCM enables to monitor the long-term (for 24 h) development of biofilm beyond the early biofilm initiation stage all the way to the mature stage. It gives the mass accumulation-induced frequency shift up to 160 KHz and the viscoelastic transitions-induced motional resistance change of 560 Ω. With the combination of both the DGTFT and QCM sensing, the full-scale and dynamic monitoring of the biofilm formation process is demonstrated; the early stage (0–5 h) by current change of DGTFT, through the growth stage (5–15 h) by motional resistance and frequency shift of QCM, to the biofilm maturation (15–24 h) by frequency shift of QCM.
Acknowledgments
This work was partially supported by the Rutgers TechAdvance® Early Technology Development grant # 205719.




