The magnetic properties of Fe-Si-B-P-C-Cu nanocrystalline powders were investigated. Fe-Si-B-P-C-Cu powders with a mostly amorphous structure were prepared by an atomization method. The atomized powders were annealed in inert gas atmosphere for nanocrystallization. The Fe84.8Si0.5B9.4P3.5C1Cu0.8 nanocrystalline powder exhibits high Bs of 1.76T, which is appreciably higher than that of conventional Fe-based amorphous powders (1.3 - 1.5 T) and close to that of Fe3.5Si4.5Cr (1.86T) crystalline one. The magnetic loss of toroidal core made of the Fe84.8Si0.5B9.4P3.5C1Cu0.8 nanocrystalline powder is also the lowest compared to that of amorphous and crystalline counterparts. This result indicates that Fe-Si-B-P-C-Cu nanocrystalline powders could contribute in efficiency-improvement as well as miniaturization of soft-magnetic cores.
I. INTRODUCTION
Energy saving is one of the most important issue on a global basis. In the regard of electric-generating facility, the improvement of power transformation efficiency is strongly required. Soft magnetic alloys have been widely used for transformers and motors as magnetic-core materials. Especially, the production of soft magnetic powders (Fe-Si, Fe-Si-Cr, amorphous alloy) is rapidly becoming larger. Fe-Si(-Cr) powders have a high saturation magnetic flux density Bs and are generally used for high-power devices. However, high coercivity of Fe-Si(-Cr) alloys causes large energy loss which degrades power-transformation efficiency. On the other hand, Fe-based amorphous powders have ever been expected to improve the efficiency of magnetic-core materials since they show very low core loss. However, the low saturation magnetic flux density (Bs) of Fe-based amorphous alloys, for example, it is only as low as 150 emu/g (1.34T) for (Fe0.97Cr0.03)76(Si0.5B0.5)22C2 amorphous powder, causes limitation on a maximum allowable current.1 Nanocrystallization of amorphous state is also one of the most effective methods to improve the soft magnetic properties. “FINEMET” (Fe-Si-B-Nb-Cu) is a well-known industrial nanocrystalline material which shows very low core loss.2 A microstructure consisting of very fine α-Fe phase embedded in amorphous matrix can be obtained by a suppression of long distance diffusion by Nb atom during a heat treatment.3 However, the saturation magnetic flux density was still very low because of the Nb addition.
Recently, Makino, et al. have developed Fe-Si-B-P-Cu nanocrystalline alloy named NANOMET® which exhibited appreciably high Bs and low core loss.4 The nanocrystalline structure of NANOMET® is developed by annealing amorphous state of precursor samples. The nanocrystallization mechanism of “NANOMET® ” has been reported in by Takeuchi, et al.5 and Sharma, et al.6 Presently, most NANOMET® samples are in the form of ribbon which are produced by a melt spinning method.7 This technique is reliable to obtain good-quality amorphous precursors because of its high quenching rate. The properties of ribbon wound cores has been reported by Takenaka, et al.8
Meanwhile, soft-magnetic powders are widely used for high frequency applications because of its low eddy-current loss.9 Nowosielski, et al. have reported a pulverization technique using a combined process of thermal nanocrystallization and high-energy ball milling applied to Fe-based amorphous ribbons.10 The obtained powders, however, had shape like flakes or plates with sharp edges. In geometric perspective, nearly spherical shaped particles are preferable in forming high-density core samples. Gas-atomization may be one of proper techniques which can produced spherical powder particles, but this method has limitation in quenching rate compared to melt-spinning.11 In this work, we reported investigation on magnetic properties of Fe-Si-B-P-Cu NANOMET® powders produced by spinning-water atomization technique. This atomization method was reported to result in higher quenching rate than the conventional gas-atomization.1
II. EXPERIMENTAL PROCEDURES
Fe83.3Si4B8P4Cu0.7 and Fe84.8Si0.5B9.4P3.5C1Cu0.8 alloy ingots were prepared by induction melting in argon atmosphere. Powder samples were prepared by a spinning water gas-atomizing technique using argon jet-gas with a setting pressure of 8 - 9 MPa. Such high gas ejection pressures were required to produce sufficiently fine and spherical molten droplets prior to subsequent quenching by high-pressure spinning water. The powders were then sieved to select the finest particles (<20 in average diameter) which are believed to undergo sufficient quenching rate to form amorphous powders. The as-atomized powders (<20 in size) were annealed at various temperatures in argon atmosphere by an infrared image furnace to develop nanocrystalline structure. The heating rate was 6.7K/s.
The morphology of the as-atomized powder was observed by scanning electron microscopy (SEM). The structure of as-atomized and annealed powders was identified by X-ray diffractometer (XRD) and transmission electron microscopy (TEM). A saturation magnetic flux density was measured by a vibrating sample magnetometer (VSM) under a maximum applied field of 12 kA/m. Powders were formed in a square shape with dimension of 7 mm x 7 mm. The specific density was measured by an Archimedes method.
Toroidal cores with outer diameter of 13 mm, inner diameter of 8 mm and height of 3.1 – 3.5 mm were prepared by pressing the powders which were previously mixed and consolidated with phenol resin (3 wt.%) and lubricant (1 wt.%). The applied pressure was 1500 MPa and then curing was applied at 160oC following the consolidation. A copper wire with 0.3 mm in diameter was wound surrounding the cores with 32 turns. The permeability was then measured by impedance analyzer (Agilent, HP4294A). The core loss Pcv was measured by B-H analyzer (IWATSU, SY8212) at 100 kHz under 100 mT.
III. RESULTS AND DISCUSSION
FIG. 1 shows an SEM image of the Fe84.8Si0.5B9.4P3.5C1Cu0.8 as-atomized powder. It is clearly seen that the particle shape is nearly spherical. A few satellite particles (a small particle attached on a surface of large particle) were also seen in FIG. 1. It is suggested that an early solidified small particle may collide with a large molten droplet during atomization. Such nearly spherical powders without appreciably amount of planar, edgy or spongy shaped particles are proper enough for making high-density toroidal cores and to examine the core performance. FIG. 2 shows XRD patterns of as-atomized and annealed Fe83.3Si4B8P4Cu0.7 powders at different annealing temperatures (Ta). A typical broad pattern of amorphous structure and weak diffraction peaks of α-Fe phase were observed in as-atomized powder indicating that it is composed of mostly amorphous phase and small amount of α-Fe crystalline phase. After annealing at 723 K, the diffraction intensity of α-Fe became stronger and subsequently after annealing at 798 K, some additional diffraction peaks (which are identified to be belonged to Fe-B phase) were observed. FIG. 3(a) shows a TEM image of the Fe84.8Si0.5B9.4P3.5C1Cu0.8 as-atomized powder. The image shows that a small amount of very fine α-Fe grain was observed in addition of mostly amorphous structure. This TEM result is in good agreement with the XRD pattern in FIG. 2. FIG. 3(b) and (c) show respectively high-angle annular dark-field scanning-transmission electron microscopy (HAADF-STEM) and bright field TEM images of a Fe84.8Si0.5B9.4P3.5C1Cu0.8 powder after annealing at 743K. The image revealed a structure consisting of fine crystal grains embedded in amorphous phase (FIG. 3(b)). However, some coarse grains were also observed as shown in FIG. 3(c). It is suggested that α-Fe grains formerly nucleated in the as-atomized state have grown by annealing.6
The scanning electron microscopy image of as-atomized Fe84.8Si0.5B9.4P3.5C1Cu0.8 powder with average size of <20 in diameter.
The scanning electron microscopy image of as-atomized Fe84.8Si0.5B9.4P3.5C1Cu0.8 powder with average size of <20 in diameter.
The XRD patterns of as-atomized and annealed powders of Fe83.3Si4B8P4Cu0.7 alloy.
The XRD patterns of as-atomized and annealed powders of Fe83.3Si4B8P4Cu0.7 alloy.
(a) A TEM image of Fe84.8Si0.5B9.4P3.5C1Cu0.8 as-atomized powder. HAADF-STEM (b) and bright field (c) images of Fe84.8Si0.5B9.4P3.5C1Cu0.8 powder after annealing at 743 K.
(a) A TEM image of Fe84.8Si0.5B9.4P3.5C1Cu0.8 as-atomized powder. HAADF-STEM (b) and bright field (c) images of Fe84.8Si0.5B9.4P3.5C1Cu0.8 powder after annealing at 743 K.
FIG. 4 shows the correlation of saturation magnetic flux density Bs with the amount of Fe content of several soft magnetic powders. In general, the Bs of amorphous alloys mainly depends on a content of the ferromagnetic elements and its magnetic moment per an atom. The Bs of Fe83.3Si4B8P4Cu0.7 and Fe84.8Si0.5B9.4P3.5C1Cu0.8 nanocrystallized powders annealed at 743K were 1.71 T and 1.77 T respectively, which are appreciably higher than that of amorphous powders (1.34 T - 1.49 T). As shown in FIG. 4, Bs of amorphous powders increased proportionally with Fe content. But, the Bs of nanocrystalline powders were not found in the extension line of Bs–Fe-content plot of amorphous powders. In fact, there is an abrupt increase of Bs at Fe content about 85 at.% which indicates the significant effect of nanocrystallization on the Bs. In the case of Fe84.8Si0.5B9.4P3.5C1Cu0.8, Bs increased by 6% by annealing at 743 K and reached the value close to that of the commercial Fe88.6Si6.7Cr4.7 crystalline powder (1.86 T).
Change in saturation magnetic flux density Bs of nanocrystalline and amorphous powders with Fe content. The data of a commercial crystalline powder (Fe88.6Si6.7Cr4.7) is also included for comparison.
Change in saturation magnetic flux density Bs of nanocrystalline and amorphous powders with Fe content. The data of a commercial crystalline powder (Fe88.6Si6.7Cr4.7) is also included for comparison.
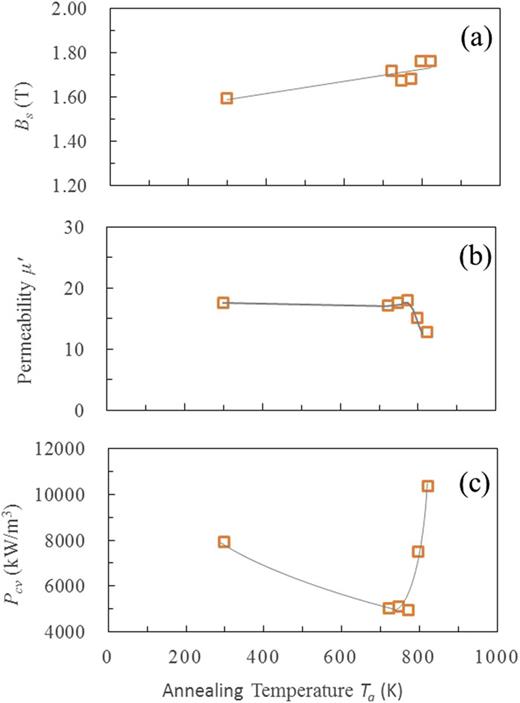
FIG. 5(a) shows the variation of Bs with annealing temperature Ta. Bs increased by annealing at 723 K – 823 K with increasing the amount of α-Fe phase. The permeability was almost constant in the region of Ta<773K, but decreased abruptly for Ta >798 K (FIG. 5(b)). This can be explained by the fact that annealing at Ta > 798 K causes the precipitation of Fe-B and other phases which do not possess ferromagnetic property. On the other hand, the core loss, Pcv showed minimum at Ta = 723 K – 773 K (FIG. 5(c)). Subsequently, Pcv drastically increased for Ta > 773 K due to the same reason as for the abrupt decrease of .
Change in the magnetic properties of Fe83.3Si4B8P4Cu0.7 powders and cores with annealing temperature Ta: (a) saturating magnetic flux density Bs of powders, (b) permeability of toroidal cores, (c) core loss Pcv of toroidal cores at 100 kHz under 100 mT. The data at Ta = 300K means an as-atomized powder.
Change in the magnetic properties of Fe83.3Si4B8P4Cu0.7 powders and cores with annealing temperature Ta: (a) saturating magnetic flux density Bs of powders, (b) permeability of toroidal cores, (c) core loss Pcv of toroidal cores at 100 kHz under 100 mT. The data at Ta = 300K means an as-atomized powder.
FIG. 6 shows the plot of Pcv of several soft-magnetic powder cores and their Bs in powder form. Both Pcv and Bs of Fe83.3Si4B8P4Cu0.7 and Fe84.8Si0.5B9.4P3.5C1Cu0.8 nanocrystalline powders are appreciably lower and higher, respectively than those of amorphous counterparts indicating the superior soft-magnetic properties of NANOMET® powders. In addition, the Fe84.8Si0.5B9.4P3.5C1Cu0.8 nanocrystalline powder exhibits lower Pcv than that of the Fe88.6Si6.7Cr4.7 commercial crystalline powder regardless the lower Bs. Bs of NANOMET® powders, however, can actually be increased if the initial structure of the powder precursors is fully amorphous. For comparison, nanocrystalline ribbons of the Fe84.8Si0.5B9.4P3.5C1Cu0.8 NANOMET® alloy obtained by annealing fully-amorphous melt-spun ribbon samples, exhibited Bs of 1.83 T7 which is much higher than that of powders (1.77 T). The not-fully amorphous structure in which a small amount of α-Fe phase has already nucleated is possibly the reason for the present Fe84.8Si0.5B9.4P3.5C1Cu0.8 powders for not being able to achieve the best Bs value. In fact, the pre-existing crystal grains make the immediate residual amorphous phase more stable and prevent the increase of volume fraction of α-Fe phase during annealing.6 The improvement of the powder preparation technique with high quenching rate to produce fully-amorphous powders then becomes the next challenge.
Plot of Pcv of soft magnetic powder cores (at 100 kHz, under 100 mT) vs. Bs of soft magnetic powders. The data of a commercial crystalline powder (Fe88.6Si6.7Cr4.7) is also included for comparison.
Plot of Pcv of soft magnetic powder cores (at 100 kHz, under 100 mT) vs. Bs of soft magnetic powders. The data of a commercial crystalline powder (Fe88.6Si6.7Cr4.7) is also included for comparison.
IV. CONCLUSION
The magnetic properties of Fe-Si-B-P-C-Cu nanocrystalline (NANOMET® ) powders were investigated. The saturation magnetic flux density increased by nanocrystallization and achieved as high as 1.76 T which is close to that of the Fe88.6Si6.7Cr4.7 commercial crystalline powder. The Fe-Si-B-P-C-Cu nanocrystalline powders also exhibit appreciably low core loss. In particular, the core loss of Fe84.8Si0.5B9.4P3.5C1Cu0.8 nanocrystalline powders is the lowest one compared to that of the amorphous and crystalline counterparts presented in this work. As the combination of low core loss and high Bs is crucial for energy saving and product-miniaturization, the results indicate the great applicability of NANOMET® powders for high-efficiency soft-magnetic core materials.
ACKNOWLEDGMENTS
This work was supported by “Tohoku Innovative Materials Technology Initiatives for Reconstruction (TIMT)” funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Reconstruction Agency, Japan. The authors thank Dr. M. Nishijima for TEM observations. NANOMET® is a registered trademark of NEC TOKIN Corporation and Tohoku Magnet Institute Co., Ltd.